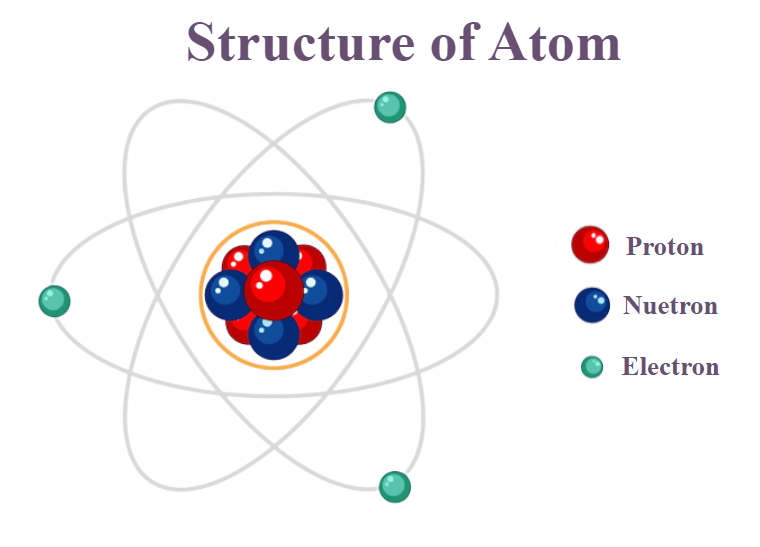
The atomic theory of matter was first proposed by John Dalton. Fundamental particles of an atom are Electron, Proton and Neutron.
Proton(p): Discovered by E.Goldstein.
- Protons are positively charged. 2. The absolute charge on the electron to be+1.6×10–19C.
Electron (e): Discovered by J.J. Thomson when he was studying the properties of cathode ray.
- Irish physicist George Johnstone Stoney named this charge ‘electron’ in 1891.
- Electrons are negatively charged.
- The absolute charge on the electron to be –1.6×10–19C.
- e/me as:=1.758820×1011Ckg–1
- The charge of an electron was measured by R.Millikan in Oil drop experiment.
- The mass of a neutron is taken as one unit each
Atomic nucleus–Discovered by E.Rutherford
- The fast moving alpha(α)-particles(doubly-charged helium ions) were made to fall on a thin gold foil.
- The mass of an atom is the sum of the masses of protons and neutrons present in the nucleus.
Valency
- The number of electrons gained, lost or shared so as to make the octet of electrons in the outermost shell, is called valency.
- The atoms of elements, having a completely filled outermost shell show little chemical activity, their valency is zero.
- An outermost-shell, which had eight electrons is said to possess an octet. Atoms would thus react, so as to achieve an octet in the outermost shell.
- The chemical behavior of an atom depend upon the number of electrons orbiting around the nucleus.
Atomic Number
The atomic number is defined as the total number of protons present in the nucleus of an atom. It is denoted by “Z”.
Mass number
The mass number is defined as the sum of the total number of nucleons (protons and neutrons) present in the nucleus of an atom.
Isotopes
- Atoms which have the same atomic number but different mass numbers. The chemical properties of isotopes are similar but their physical properties are different. But some isotopes have special properties which find them useful in various fields. Some of them are :
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goiter.
Radioactive isotopes
Arsenic–74→detect tumors Sodium–24 → Blood clot Iodine–131 →Activity of thyroid gland Cobalt–60 → Treat of cancer
Isobars -Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
Isotones–Atoms having same number of neutrons.
Isoelectronics–Isoelectronic refers to two atoms, ions or molecules that have the same electronic structure and same number of valence electrons.
Mass defect – The mass defect is the difference between the rest mass of a nucleus and the sum of the rest masses of its constituent nucleons.
Featured image courtesy: geeksforgeeks