Physical Change
- The change that only affect physical properties, but the chemical compositions remains unchanged, are called physical change.
- These can be reversed by changing the conditions of temperature and pressure, boiling, cutting of trees, dissolving common salt in water burning of wax.
Chemical Change
- The change which affect the composition as well as chemical properties of matter and result in the formation of a new substance is called a chemical change.
- Chemical changes are generally irreversible. Some examples of chemical changes are burning of candle (gases), photosynthesis, ripening of fruits, electrolysis of water.
- A chemical reaction involves bond breaking or bond formation between any two atoms to produce new substances.
Laws of Chemical Combination -There are three laws of Chemical combination. They are:
- Law of conservation of mass: This law was stated by Lavoisier in 1744. It states that “In all physical and chemical changes, the total mass of reactants is equal to total mass of products.”
- Law of constant proportions (or constant composition): This law was first stated by Proust in 1797. According to the law “a chemical compound is always found to be made up of the same elements combined together in the same proportions by weight” e.g. the ratio of hydrogen and oxygen in pure water is always 1:8 by weight. This law is also called law of definite proportions.
- Law of multiple proportions: This law was given by John Dalton (1803) and states that “when two elements combine to form two or more compounds, the different mass of one of the elements and the fixed mass of the one with which it combines always form a whole number ratio”. This law explains the concept of formation of more than one compound by two elements.
Types of Chemical Reactions.
Exothermic and Endothermic Reactions
Reactions in which heat is released along with the formation of products, are called exothermic reactions. Burning of fuel is an example of exothermic reaction.
Reactions in which heat is absorbed, are known as
Endothermic reactions.
Oxidation and Reduction
Oxidation is removal of electrons.
Reduction is the addition of electrons.
Oxidation means
- Addition of oxygen
- Removal of hydrogen.
Reduction means
- Removal of oxygen.
- Addition of hydrogen.
The substance that causes oxidation is called the oxidizing agent.
The substance that causes reduction is called the reducing agent.
Oxidising agent
- Acceptors of electrons.
- It is a substance which removes the electron from an atom.
- It brings about oxidation.
Reducing agent
- Donors of electrons.
- It is a substance which adds electrons to an atom.
- It brings about reduction.
REDOX REACTION
A reaction which involves oxidation and reduction occurring simultaneously together are called redox reaction. Photosynthesis in plants digestion of food in animals; dry and wet batteries and corrosion of metals are diverse examples of oxidation and reduction reactions.
Electrolysis
- Electrolysis is carried out in an electrolytic cell.
- A simple electrolytic cell consists of two copper strips dipping in an aqueous solution of copper sulphate.
- On applying DC voltage to the two electrodes, copper metal is deposited on cathode and copper is dissolved at anode.
- Used In the purification of impure metals.
- In the extraction of metals
- The blocks used in typing industries are prepared by electrolysis.
- Steel is coated with zinc metal during the process of galvanization.
Batteries
These convert chemical energy into electrical energy. Mainly two types of batteries are used, i.e. primary and secondary.
Primary Batteries
In the primary batteries, reaction occurs only once and after a period of time battery becomes dead.
Dry Cell or Leclanche Cell
It consists of a zinc container that acts as anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon.
A moist paste of ammonium chloride (NH4CI) and zinc chloride (ZnCI2) is used as an electrolyte. Dry cell is commonly used in our transistors and clocks.
Mercury Cell
It is commonly used in low current devices such as hearing aids, watches etc.
The electrolyte is a past of potassium hydroxide(KOH)and zinc oxide (ZnO).
Secondary Batteries Lead Storage Battery
It consists of a lead as anode and a grid of lead packed with lead dioxide (PbO2) as cathode.
A 38% solution of sulphuric acid is used as an electrolyte. On charging the battery, the reaction is reversed and lead sulphate gives lead on anode and cathode is converted into lead dioxide respectively.
Nickel Cadmium Cell
It has longer life that the lead storage cell. It consists of a cadmium as anode and nickel dioxide as cathode. The electrolyte is a potassium hydroxide (KOH) solution.
Fuel Cells
Fuel cells convert energy from the combustion of fuels such as hydrogen, carbon monoxide, methane directly into electrical energy
A fuel cell with hydrogen and oxygen has been used for electric power in Apollo Space Programme.
Corrosion
- When iron is exposed to moist air for a long period of time, its surface acquires a coating of brown flaky substance called rust.
- Rust is mainly hydrated iron(III)oxide (Fe2O3.xH2O).
- In corrosion, a metal is oxidized by the loss of electrons to oxygen and form oxide.
- The rusting of iron can be prevented by painting, oiling and greasing, galvanizing (by coating iron objects with zin(c), chrome plating etc.
Catalysis
- A catalyst is a substance which alter the rate of reaction.
- The catalyst itself does not alter during the reaction.
- The phenomena in which the rate of reaction is altered by the presence of a substance (catalyst) is known as catalysis.
- Catalysts are specific in their action.
- A catalyst does not change the equilibrium state of a reversible reaction, only brings it quickly.
- The main function of a catalyst in a reaction is to decrease the activation energy.
Applications of Catalysts in Industrial Processes
- Haber process for ammonia—Iron is used as a catalyst and molybdenum is used as a promoter of catalyst iron.
- Contact process for Sulphuric acid—Vanadium pentoxide is used as a catalyst.
- Ostwald process for Nitric acid—Platinum gauze is used as a catalyst.
- Deacon process for Chlorine—Cupric chloride is used as a catalyst.
- Synthesis of petrol—Nickel, iron, cobalt and alumina is used as a catalyst.
Enzyme Catalysis
The increase in the rate of reaction by the enzymes is known as enzyme catalysis. They are biocatalysts, all are proteins in nature.
The rates of enzymatic reactions are very much affected by pH change.
Some important enzyme catalysis reactions are as follows:
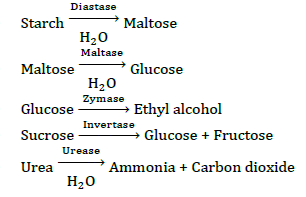
Featured image courtesy: sssi